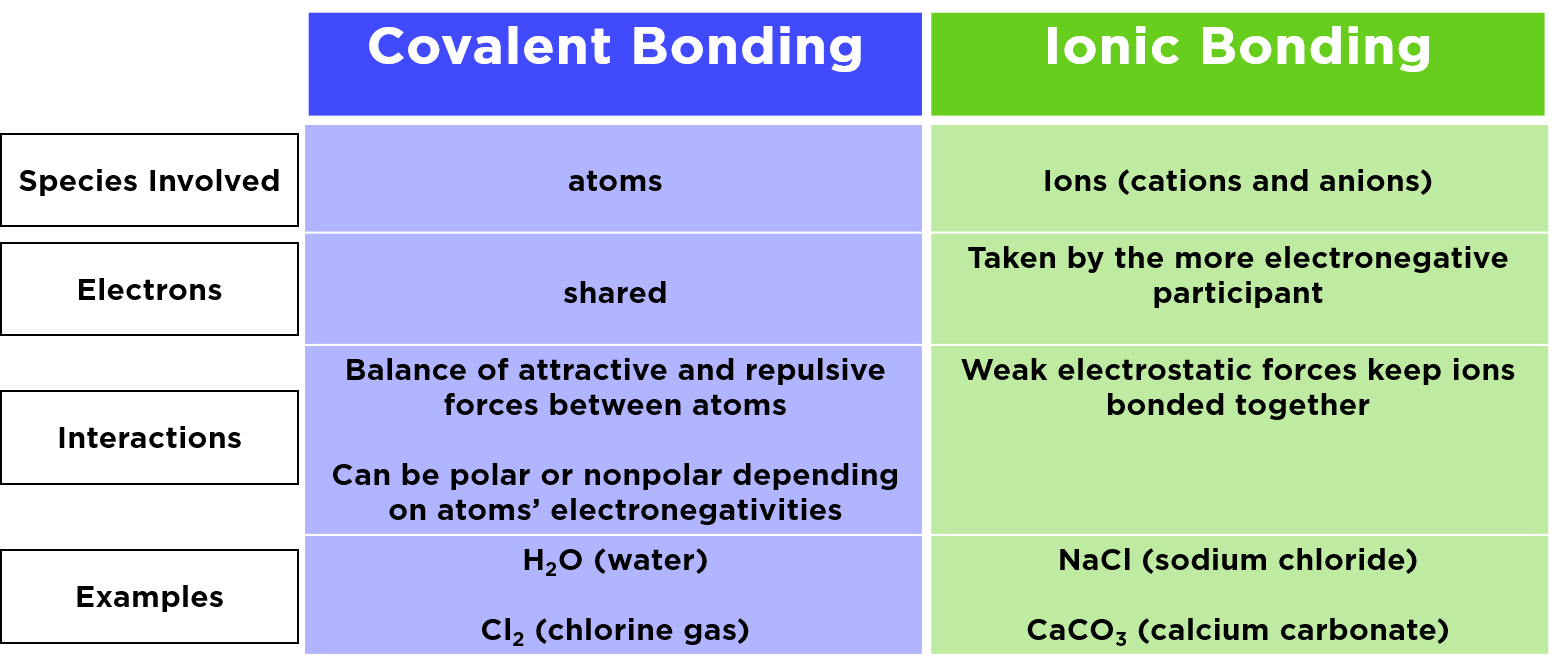
Are Ionic Bonds Metallic . The simplest model of metallic. The atom that gains electrons becomes a negatively. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. By definition, a metal is relatively stable if it loses electrons to form a. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Web the atom that loses electrons becomes a positively charged ion, known as a cation.
from www.expii.com
Ionic bonds are formed between cations and anions. The atom that gains electrons becomes a negatively. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. By definition, a metal is relatively stable if it loses electrons to form a. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. The simplest model of metallic. Web the atom that loses electrons becomes a positively charged ion, known as a cation.
Ionic Bonding (Biology) — Definition & Role Expii
Are Ionic Bonds Metallic Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a. Ionic bonds are formed between cations and anions. Web the atom that loses electrons becomes a positively charged ion, known as a cation. The atom that gains electrons becomes a negatively. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. The simplest model of metallic.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Are Ionic Bonds Metallic Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. The atom that gains electrons becomes a negatively. The simplest model of metallic. Ionic bonds are formed between cations and anions. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. A cation is formed. Are Ionic Bonds Metallic.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID6126176 Are Ionic Bonds Metallic Web the atom that loses electrons becomes a positively charged ion, known as a cation. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. The simplest model of metallic. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion is formed when. Are Ionic Bonds Metallic.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet Are Ionic Bonds Metallic A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Web the atom that loses electrons becomes a positively charged ion, known as a cation. The atom that gains electrons becomes a negatively.. Are Ionic Bonds Metallic.
From www.pinterest.com
Metallic Bond A bond formed when metal atoms share electrons. school Are Ionic Bonds Metallic Ionic bonds are formed between cations and anions. Web the atom that loses electrons becomes a positively charged ion, known as a cation. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior. Are Ionic Bonds Metallic.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Are Ionic Bonds Metallic Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Web the atom that loses electrons becomes a positively charged ion, known as a cation. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Ionic bonds are formed between cations and anions. A cation. Are Ionic Bonds Metallic.
From www.nagwa.com
Question Video Identifying the Structure of a Metallic Bond Nagwa Are Ionic Bonds Metallic The atom that gains electrons becomes a negatively. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Ionic bonds are formed between cations and anions. The simplest model of metallic. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a. Are Ionic Bonds Metallic.
From studylib.net
Lesson 3 Ionic and Metallic Bonds Are Ionic Bonds Metallic Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. The simplest model of metallic. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Web the atom that loses electrons becomes a positively charged ion, known as a cation.. Are Ionic Bonds Metallic.
From exoplzjjb.blob.core.windows.net
Ionic Bond Definition Vs Covalent at Dennis Wells blog Are Ionic Bonds Metallic The atom that gains electrons becomes a negatively. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Ionic bonds are formed between cations and anions. The simplest model of metallic. A cation is formed when a metal. Are Ionic Bonds Metallic.
From www.youtube.com
Types of Bonding (Ionic, Covalent, Metallic) GCSE Chemistry Revision Are Ionic Bonds Metallic By definition, a metal is relatively stable if it loses electrons to form a. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Ionic bonds are formed between cations and anions. Web the atom that loses electrons becomes a positively charged ion, known as a cation. The atom that gains electrons becomes a negatively. The. Are Ionic Bonds Metallic.
From pediaa.com
Difference Between Ionic Covalent and Metallic Bonds Definition Are Ionic Bonds Metallic Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Ionic bonds are formed between cations and anions. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. The simplest model of metallic. Web metallic bonding is sort of like covalent. Are Ionic Bonds Metallic.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Are Ionic Bonds Metallic Web the atom that loses electrons becomes a positively charged ion, known as a cation. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. The simplest model of metallic. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. The atom that gains electrons becomes a negatively.. Are Ionic Bonds Metallic.
From animalia-life.club
Metallic Bond Examples List Are Ionic Bonds Metallic Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. The simplest model of metallic. A cation is formed when a metal ion loses a valence electron. Are Ionic Bonds Metallic.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Are Ionic Bonds Metallic Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure. Web the atom that loses electrons becomes a positively charged ion, known as a cation. The simplest model of metallic. Ionic bonds are formed between cations and anions. Web ionic bonding results in compounds known as ionic, or electrovalent,. Are Ionic Bonds Metallic.
From kemrennmcmehon.blogspot.com
Ionic and Covalent Bonds KemrennMcmehon Are Ionic Bonds Metallic The atom that gains electrons becomes a negatively. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron. Are Ionic Bonds Metallic.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Are Ionic Bonds Metallic The atom that gains electrons becomes a negatively. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Web metallic bonding is sort of like covalent bonding, because it involves sharing electrons. Web the atom that loses electrons becomes a positively charged ion, known as a cation. Web alloys are commonly used. Are Ionic Bonds Metallic.
From en.wikipedia.org
Ionic bonding Wikipedia Are Ionic Bonds Metallic The atom that gains electrons becomes a negatively. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. By definition, a metal is relatively stable if it loses electrons to form a. Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Ionic. Are Ionic Bonds Metallic.
From impressedselect.blogspot.com
which hybridization scheme allows the formation of at least one π bond Are Ionic Bonds Metallic Web ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Web the atom that loses electrons becomes a positively charged ion, known as a cation. The atom that gains electrons becomes a negatively. A cation is formed when a metal ion loses a valence electron while an anion is formed when a.. Are Ionic Bonds Metallic.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Are Ionic Bonds Metallic By definition, a metal is relatively stable if it loses electrons to form a. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Ionic bonds are formed between cations and anions. Web alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to. Are Ionic Bonds Metallic.